Inflammasomes and Pyroptosis Pathways
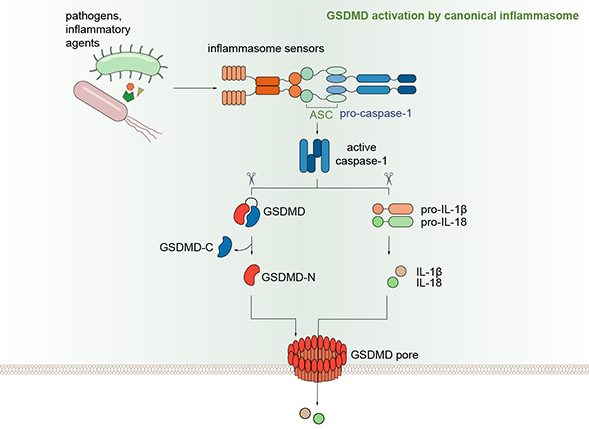
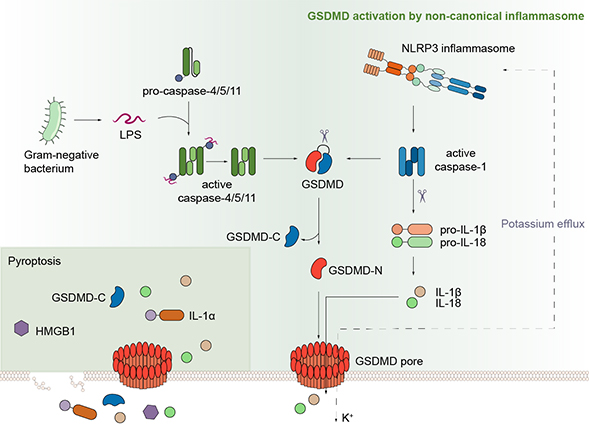
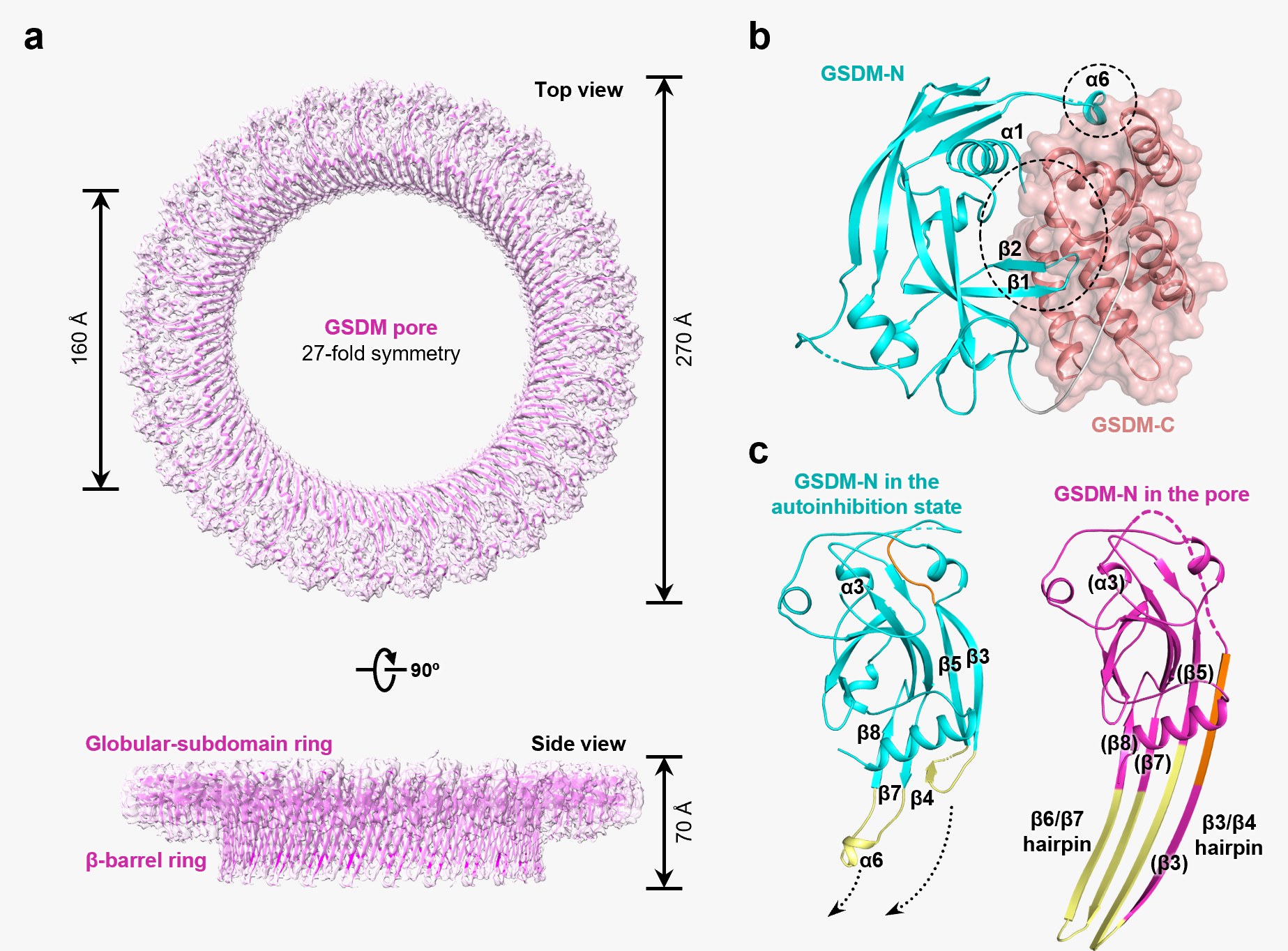
Pyroptosis, a novel necrotic form of programmed cell death, is characterized by continuous swelling of cells until the cell membrane ruptures, resulting in the release of cellular contents and subsequent activation of a strong inflammatory response. In 2015, Dr. Shao found that GSDMD is the pyroptosis executor downstream of both caspase-1 and caspase-11/4/5. Inter-domain cleavage of GSDMD by either caspase-1 or caspase-11/4/5 allows the N-terminal domain to bind membrane phospholipids and oligomerize into a large ~18-nm pore in the plasma membrane, causing changes in cell osmotic pressure and swelling until the final rupture of the cell membrane (Shi et al., Nature 2015; Ding et al., Nature 2016), as well as the release of various pro-inflammatory cytokines, including IL-1β.
Pyroptosis plays an important role in combatting infection and limiting the growth of cancerous cells. Excessive or inhibited pyroptosis is implicated in nervous system diseases, infectious diseases, autoimmune diseases, cardiovascular disease, and tumor growth. The in-depth study of pyroptosis will help to understand its role in the occurrence and outcome of related diseases, and provide new ideas for clinical prevention and treatment.
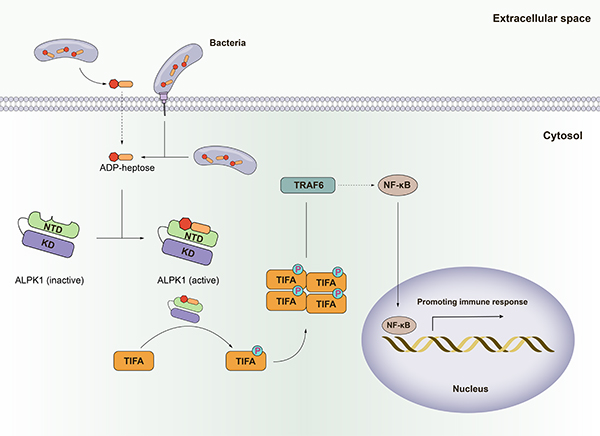
Dysregulated inflammasome signaling can lead to hyper-inflammation in response to environmental triggers, contributing to the pathogenesis of inherited auto-inflammatory diseases. Gain-of-function mutations in NLRP3 and MEFV genes have been found to cause Cryopyrin-Associated Periodic Syndromes (CAPS) and Familial Mediterranean fever (FMF), respectively. NLRP3 has become a major target for drug development. However, GSDMD, which is required for all NLRP3 signaling, may potentially be an alternate or more attractive drug target than direct inhibition of NLRP3.
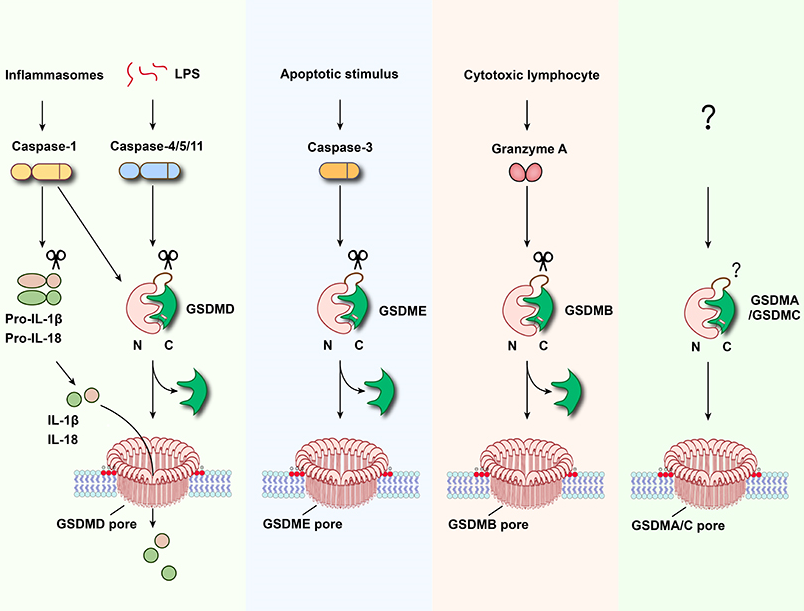
The gasdermin family consists of GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and PJVK. The N-terminal domains of all gasdermin members (except PJVK) can oligomerize and form membrane-spanning pores, ultimately resulting in pyroptosis(Shi et al., Nature 2015, Ding et al., Nature 2016). Studies have shown that gasdermin family proteins play key roles in sepsis, multiple sclerosis, asthma, adverse effect of chemotherapy drugs, cytokine storm caused by virus infection or CAR-T therapy, and inflammatory diseases such as inflammatory bowel disease (IBD). Therefore, the development of highly selective, potent drugs that inhibit gasdermins will provide new solutions to address unmet clinical needs.
There is growing evidence that pyroptosis-induced inflammation significantly alters the tumor immune microenvironment, transforming ''cold'' tumors into ''hot'' tumors, enabling tumor cells to enhance their ability to response to immune checkpoint inhibitors (such as anti-PD-1). Therefore, gasdermin activators may have an important role in immuno-oncology.